Prof. Dr. Andreas Meyer-Lindenberg
Department of Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University
Prof. Dr. Rolf-Detlef Treede
Department Neurophysiology, CBTM Medical Faculty Mannheim, Heidelberg University
Plasticity in brain circuits underlying interactions between pain and depression
Chronic pain and major depressive disorder (MDD) are highly prevalent conditions and frequently comorbid [Nichol et al. 2014], which complicate chronic physical disease, and both of which are leading causes of disability globally [Vos et al. 2015]. The causes of chronic pain and depression are poorly understood, although there is evidence of a contribution from genetic factors in each disorder [Diatchenko et al. 2007, Sullivan et al. 2012]. Chronic pain is caused by an accumulation of many small genetic effects (polygenic risk) and is associated with some of the same genetic and environmental risk factors that confer risk of depression [McIntosh et al. 2016].
The B09 project aims at understanding the mechanistic background of MDD being permissive of development of chronic pain diseases. It combines an array of methods, which allow comprehensive description of the functional pain phenotype of patients with MDD. To this end MDD patients will be assessed for their somatosensory perception pattern using the currently most advanced methods of quantitative sensory testing (QST [Rolke et al. 2006]). We will use various models of pain plasticity, which encompass pain summation, the experimental induction of memory-like enhanced pain sensitivity (hyperalgesia, pain long-term potentiation [Klein et al. 2004, Jürgens et al. 2014, Henrich et al. 2015): we will also assess the extinction of this increased pain sensitivity, and assess the capacity to suppress pain sensation (conditioned pain modulation). Part these tests will also be assessed in an MR scanner to understand the patterns of brain activation in patient with MDD by painful stimuli (functional MR imaging), and their distinction from heathy subjects. Assessment of executive functioning will be performed to estimate the brains ability to flexibly execute and switch its programs to adapt to varying cognitive demands. The brain activation patterns in these various experimental approaches (sensory and cognitive processing) together with data on structural properties of the patients’ brains (local brain structure and connecting fiber tracts) will be analysed for their interaction to understand how the brain areas involved in pain processing are networking. We will approach this by so-called graph theoretical analysis, which will give insight into the local and wide range interaction of brain areas (the brain connectome [Bassett et al. 2006, Erk et al. 2010, Bullmore and Bassett 2011]). To understand the specific distinctions, MDD patients will not only be compared to healthy subjects, but also to their first degree relatives (who are thought to carry a similar genetic risk structure), and to MDD patients in whom the disease has remitted either spontaneously or following treatment.
The working hypothesis assumes that a deficit in the most frontal parts of the brain (prefrontal cortex) and reduced capacity to connect widely separated brain areas through critical connecting structures (so-called “hubs”), which leads to local restriction of brain processing (so-called “small worldness” of the brain [Wang et al. 2016]) is the basis of altered processing of pain in MDD patients, and which provides the risk structure that ultimately leads to an enhanced risk to develop chronic pain disease.
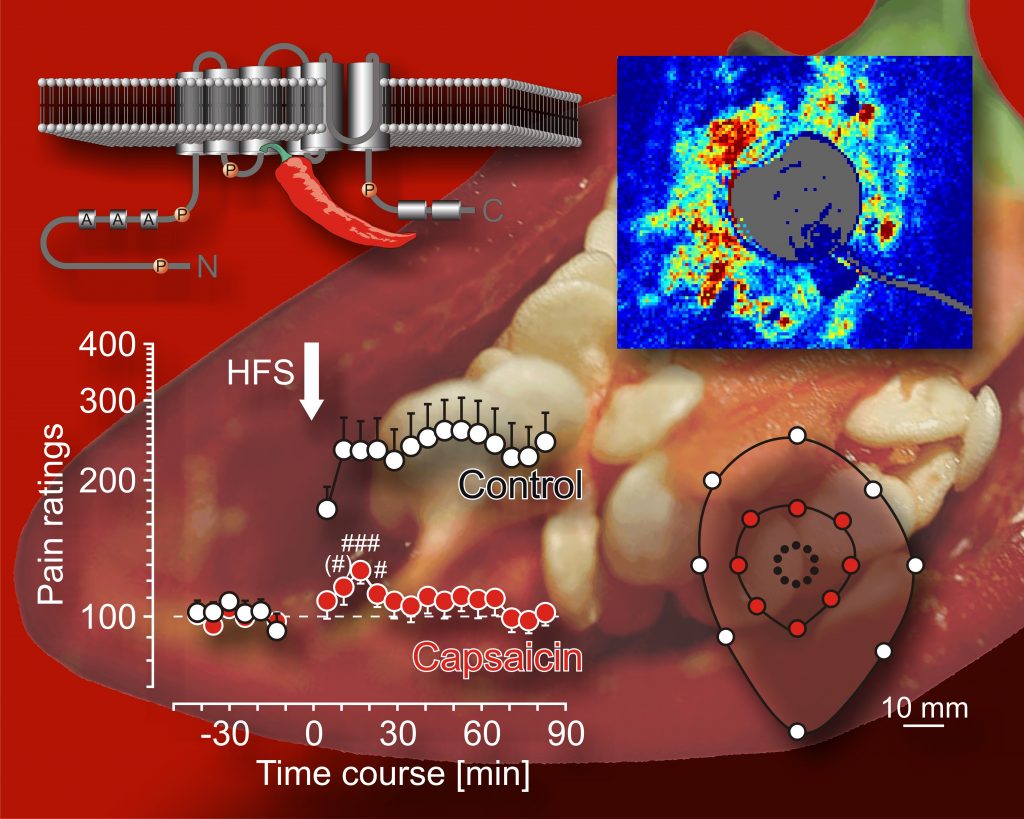
Enhanced pain sensitivity (hyperalgesia) depends on the integrity of pain sensors (nociceptors) that carry the membrane receptor TRPV1 (model of the TRPV1 receptor embedded in the cell membrane shown in upper left corner), evidence of TRPV1 activation by microvascular imaging (laser speckle imaging shown in upper right corner). Excitation by brief painful stimuli (HFS, lower left corner) elicits enhanced pain sensitivity (hyperalgesia) in normal skin (control), which extends over large skin areas (mapping of hyperalgesia, lower right corner), and which can be fully prevented temporarily by pretreatment of the nerve fibers with a specific irritant (capsaicin). Sketch based on data by Henrich et al. 2015.



















