Research Areas
A key task is to understand structure-function relations that determine sensory, emotional and cognitive processes and how these functionally integrate to form a system which underlies the highly subjective experience of acute pain. A further task is to study these in the context of chronic pain, which may involve more complex networks, comprising more ‘nodes’ and a higher degree of associations, given especially, potential, complex interactions with pathways governing emotional, motivational and cognitive processes that are also involved in psychopathological disorders, such as depression, addiction and anxiety.
To tackle these goals, a highly trans-disciplinary configuration, merging expertise in classical pain research with knowledge on other pathways, disorders and methodologies, is required. Indeed, this matches the conceptual as well as the technological repertoire of scientists working together in Heidelberg and Mannheim.
Research Area A consists of projects addressing key questions on the structure, function and specificity of distinct parts of the sensory afferent-spinal cord circuitry and the identification of genetic and epigenetic mechanisms which mediate specificity as well as structural and functional plasticity.
Peripheral Sensory Afferents
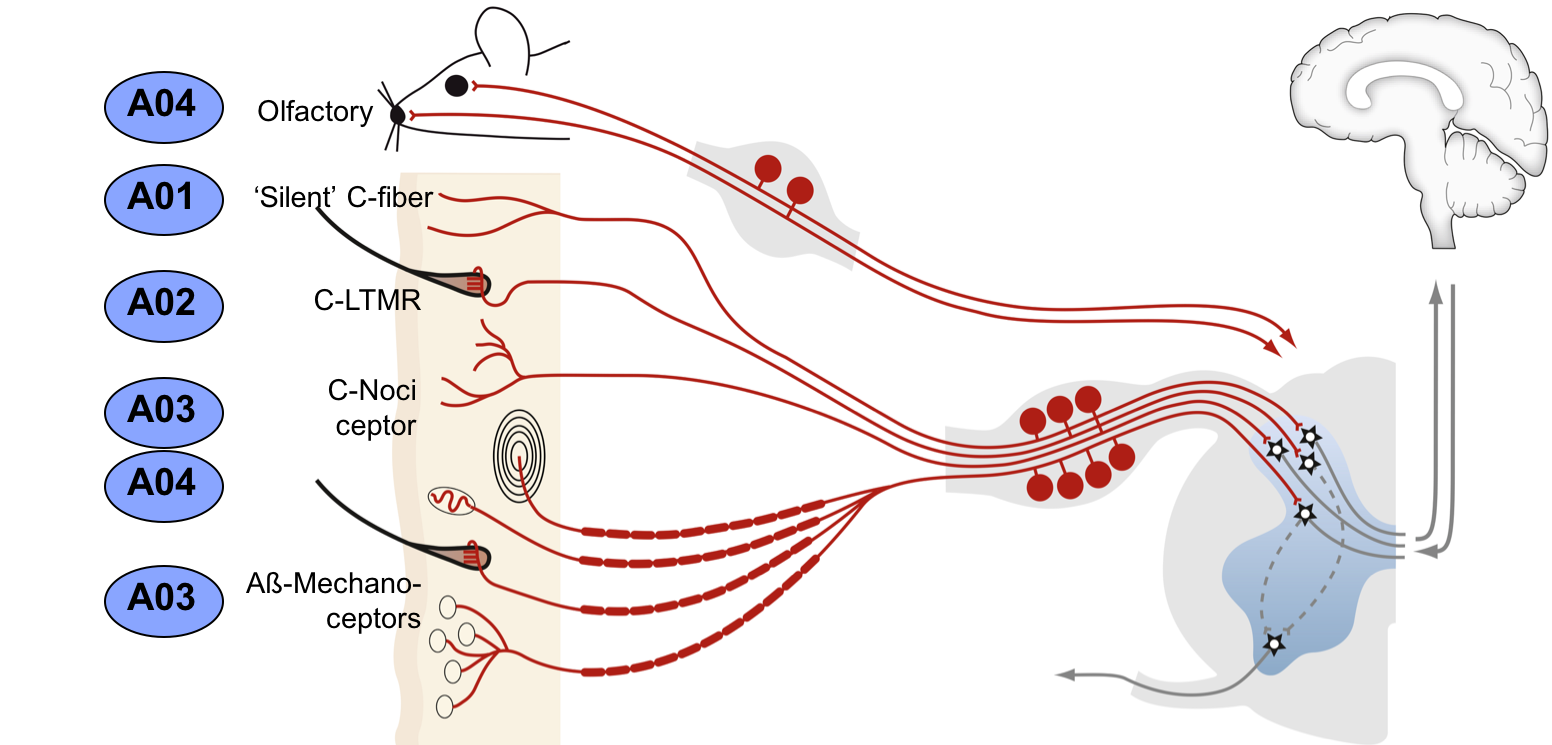
Projects A01-A04 address the functional contributions of specific afferents,
Projects focusing on elucidating structure-function relationships in distinct brain regions and circuits representing nociception and pain, their modulation by negative emotions, their
structural plasticity and their interactions with modulatory neurotransmitter systems as well as with comorbidities will be explored in Research Area B.
such as C- and A-delta nociceptors (A03 andA04), silent nociceptors (A01), C-low threshold mechanoceptors (A02) or large diameter A-beta low threshold afferents (A03). Collectively, these projects promise insights into how the peripheral inputs are orchestrated in physiological conditions and remodelled structurally and functionally over pain chronicity in a variety of models. Despite evidence for interactions, few studies have examined how nociceptive and non-nociceptive sensory circuits interact and influence one another – we therefore also aim address structural and functional convergence and crosstalk between nociceptive and non-nociceptive, e.g. olfactory and visual, signalling in the trigeminal system (A04).
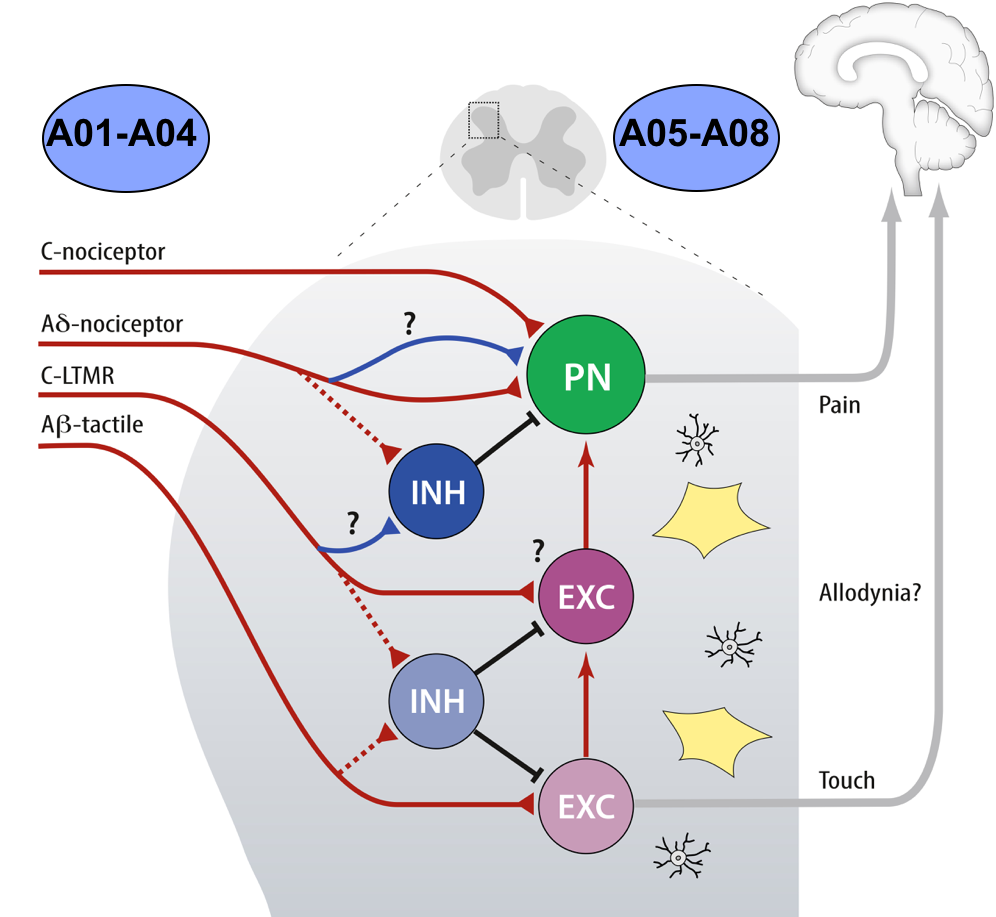
Spinal Modulation of Nociceptive Inputs
Projects A05-A08 tackle the diversity and disease-relevance of spinal neurons and their modulation by glia. Together, they promise insights into functional connectivity and specificity of recruitment of diverse types of neurons and glia (A05,A07) and nociceptive activity-dependent functional plasticity (A08) as well as central neuropathy-related structural plasticity (A06). Deciphering the specificity of genetic and epigenetic regulation in key subsets of neurons would lay an excellent basis for addressing mechanisms and identifying new therapeutic targets (A07,A08).
Pain Processing in Cortical Circuits
Projects B01-B04 address prefrontal-limbic circuits, which are particularly implicated in the emotional components of acute and chronic pain. We aim to causally address specific functional roles of individual prefrontal-limbic areas and circuits in pain chronicity in rodents and humans (B01,B03,B04),
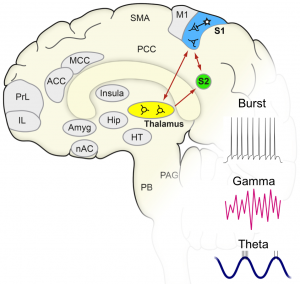
study how they are structurally and functionally modulated by stress using a longitudinal study design (B02,B03) and delineate the contribution ofdopaminergic, oxycitocinergic and glutamatergic regulatory pathways (B02,B03). On the other hand, projects B05,B06 and G01 will address the lateral somatosensory arm of nociceptive pathways and particularly analyse activity rhythms generated in the cortex during pain processing in humans and rodents. We will study the structural basis of osciallatory rhythms, such as gamma and theta band oscillations and address their functional relevance and mechanisms (B05, B06, G01), address coupling across diverse brain regions in the time and frequency domains (B05, G01) and derive the functional role of GABAergic inhibition therein (B06).
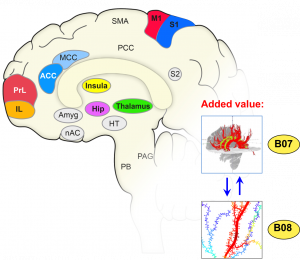
Synaptic Plasticity – Changing Shape for the worse?
Another key goal is to study maladaptive structural plasticity in cortical circuits which has been correlatively linked to chronic pain so far. Projects B07 and B08 will therefore take a close look at neocortical maladaptive structural plasticity in neuropathic pain, integrating across spatial scales, namely microscopic in animal models (B08) to macroscopic levels in humans (B07). Using a longitudinal study design, a key task to address whether maladaptive structural plasticity is a cause or consequence of chronic pain. Finally, project B09 will address a very prevalent and important comorbidity of pain, namely depression, with a central aim of addressing brain circuits mediating altered pain sensitivity in patients with major depression and to understand their plasticity over a longitudinal study.
In line with our translational focus, we have already endeavoured to match models as well as methods of analysis of nociception and pain across rodents and humans across the breadth of the consortium. This will form one of the most important tasks of the projectS01, but it also harbours a methods/model development component, which will directly aid all projects in this consortium over the first as well as future funding periods.
