Prof. Dr. Rainer Spanagel
Institute of Psychopharmacology, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University
Prof. Dr. Dr. Heike Tost
Department of Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University
Dr. Sebastian Wieland
Department of General Internal Medicine and Psychosomatics, University Hospital Heidelberg, Heidelberg University
Translational studies on the role of thalamolimbic brain circuits in the development of chronic pain and psychiatric comorbidities
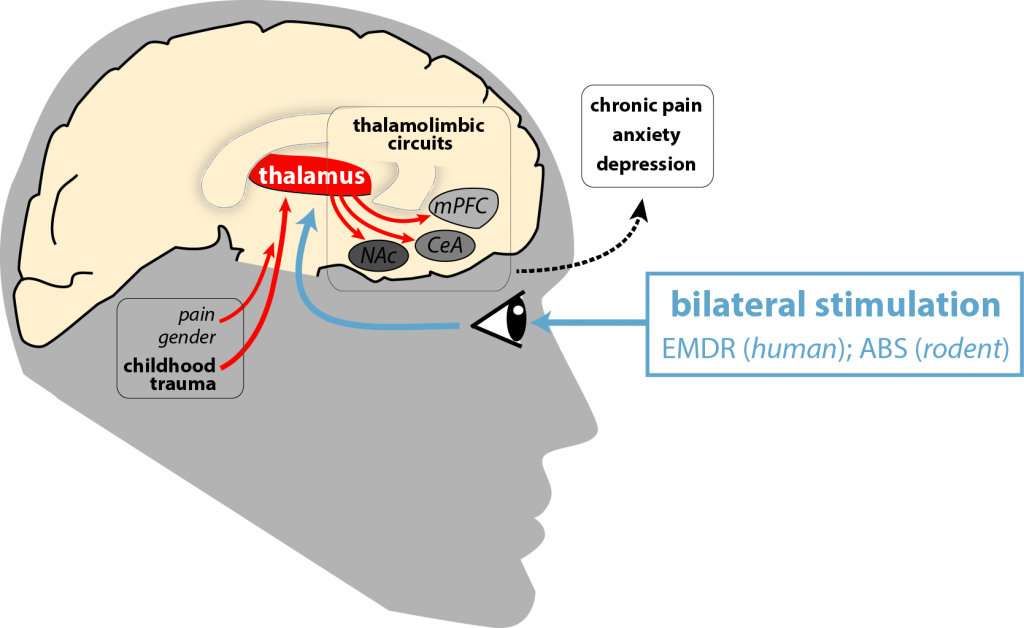
Adverse childhood experiences (ACEs) are a major risk factor for the development of chronic pain and psychiatric disorders such as anxiety and depression, especially in women. The human-rodent tandem project B04 showed that ACEs sensitize thalamolimbic circuits to facilitate chronic pain and psychiatric comorbidities in pain patients and mice.
The human part of project B04 (Tesarz, Tost) found a strong association between ACEs and psychiatric comorbidity, decreased daily well-being, and increased daily pain and affective distress in human pain patients compared to healthy controls. Brain imaging revealed significant structural and functional alterations in the thalamolimbic circuitry of pain patients, such as reduced motivational signaling in the thalamus and ventral striatum, which predicted pain severity and affective dysfunction.
The rodent part of project B04 (Spanagel, Wieland) investigated the effects of childhood social trauma on pain chronification in a two-hit mouse model and found trauma-sensitive plasticity in thalamolimbic projections causally mediating pain-associated, anxious and social behaviors, especially in female mice. We also established a novel psychotherapy-like mouse model that mimics eye movement desensitization and reprocessing (EMDR) in rodents and found that alternating bilateral stimuli (ABS) persistently reduced fear responses in traumatized mice.
We will now take a translational approach to test whether ABS can desensitize thalamolimbic circuits using in vivo fiber photometry, optogenetics, MRI, and ecological momentary assessments in mouse models and pain patients. In the long term, these findings will be translated to optimize bilateral stimulation, e.g. during EMDR psychotherapy, to improve pain relief in chronic pain patients.