Prof. Dr. Sabine C. Herpertz
Department of General Psychiatry, Centre of Psychosocial Medicine, Medical Faculty Heidelberg, Heidelberg University
Prof. Dr. Beate Ditzen
Institute of Medical Psychology, Centre of Psychosocial Medicine, Medical Faculty Heidelberg, Heidelberg University
Prof. Dr. Valery Grinevich
Department of Neuropeptide Research, Central Institute of Mental Health, Mannheim
Central oxytocin effects on acute and chronic pain processing
The hypothalamic neuropeptide oxytocin (OT) has widespread effects on human social life and has been convincingly demonstrated to act as an endogenous anaesthetic. The OT-mediated analgesia is controlled by novel, recently identified type of OT neurons exerting direct and indirect action on spinal cord sensory neurons (Eliava et al., Neuron, 2016: Figure 1).
The “pro-social” neuropeptide OT may affect pain perception via acting on high brain regions, such as limbic and cortical brain regions which are known to play a major role in the cognitive and emotional processing of pain. However, the specific OT-sensitive circuits, which modulate pain processing, as well as its effects on psychological mechanisms associated with pain perception are largely unknown. Our project is a tandem project which aims at investigating the specific role of OT in pain perception and anticipation in rodents on the one hand and humans on the other hand. In the human studies, we will employ functional magnetic resonance imaging to characterize the modulatory impact of intranasal OT on pain perception and anticipation in a Pavlovian fear conditioning task in healthy male participants. In congruency with human studies, rodents will be subjected to alternate Pavlovian fear conditioning, followed by monitoring effects of endogenous OT release within limbic and cortical regions on fear extinction. Taken together, our tandem project combines the advantages of studying OT-sensitive circuits in different species: while studies in humans permit examining the multidimensional percept of pain, rodent studies enable the tracing of OT at a high resolution and allow for testing causal relationships. Thus, our project aims at obtaining a comprehensive picture of how OT modulates pain processing and anticipation by bridging the gap between animal neurophysiology and functional imaging in humans. In a second study in humans we will further examine the role of oxytocin on the pattern of spontaneous pain in comparison with experimentally inflicted acute pain and its underlying brain circuits in chronic back pain patients.
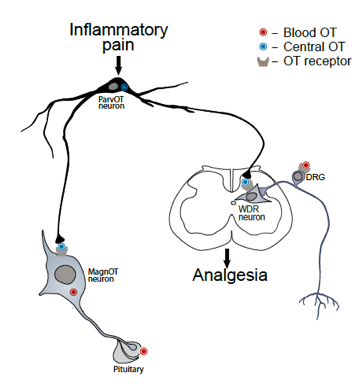
Figure 1: The role of the novel type of parvOT neurons in coordinating central and peripheral OT release to promote analgesia. We hypothesize that pain stimulates the identified subset of OT (parrvocellular, ParvOT) neurons, which induce simultaneous release of OT into systemic circulation from magnocellular OT (MagnOT) neurons and spinal cord, exerting respectively delayed and longer lasting and immediate and shorter lasting analgesia. The peripheral analgesic effect of OT is likely mediated by its action on sensory neurons of the dorsal root ganglion (DRG).